Which Of The Following Statements Is True About Modes Of Transmission For Infectious Agents?
- Review
- Open Admission
- Published:
Recognition of aerosol transmission of infectious agents: a commentary
BMC Infectious Diseases book xix, Commodity number:101 (2019) Cite this article
Abstract
Although short-range large-droplet transmission is possible for well-nigh respiratory infectious agents, deciding on whether the aforementioned agent is likewise airborne has a potentially huge impact on the types (and costs) of infection control interventions that are required.
The concept and definition of aerosols is besides discussed, as is the concept of large droplet manual, and airborne transmission which is meant by most authors to be synonymous with droplets transmission, although some utilize the term to mean either large droplet or droplets transmission.
Nonetheless, these terms are often used confusingly when discussing specific infection control interventions for individual pathogens that are accustomed to exist generally transmitted by the airborne (droplets) route (e.m. tuberculosis, measles and chickenpox). It is therefore important to clarify such terminology, where a detail intervention, like the type of personal protective equipment (PPE) to be used, is deemed acceptable to intervene for this potential mode of transmission, i.e. at an N95 rather than surgical mask level requirement.
With this in mind, this review considers the usually used term of 'aerosol transmission' in the context of some infectious agents that are well-recognized to exist transmissible via the airborne road. It also discusses other agents, similar flu virus, where the potential for airborne manual is much more dependent on various host, viral and ecology factors, and where its potential for aerosol manual may exist underestimated.
Background
The classification of an infectious amanuensis as airborne and therefore 'droplets-transmissible' has significant implications for how healthcare workers (HCWs) need to manage patients infected with such agents and what sort of personal protective equipment (PPE) they will demand to wear. Such PPE is ordinarily more than plush for airborne agents (i.due east. droplets-transmissible) than for those that are only transmitted past large droplets or directly contact considering of two key properties of aerosols: a) their propensity to follow air flows, which requires a tight seal of the PPE around the airways, and b) for bioaerosols, their pocket-sized size, which calls for an enhanced filtering capacity.
Several recent manufactures and/or guidance, based on clinical and epidemiological data, take highlighted the potential for aerosol transmission for Middle-East Respiratory Syndrome-associated coronavirus (MERS-CoV) [1, two] and Ebola virus [three, 4]. Some responses to the latter have attempted to put these theoretical risks in a more practical light [4], and this nicely illustrates the quandary of how to classify such emerging or re-emerging pathogens into either the large droplet (short-range) versus airborne (short and mayhap long-range) transmission categories. However, this delineation is non black and white, equally there is as well the potential for pathogens under both classifications to exist potentially transmitted by aerosols between people at close range (i.due east. inside i m).
Definitions
Strictly speaking, 'aerosols' refer to particles in suspension in a gas, such as small droplets in air. At that place have been numerous publications classifying aerosol using particle sizes over the years [5,6,7,8,9,10]. For case it is generally accustomed that: i) pocket-size particles of < 5–10 μm aerodynamic diameter that follow airflow streamlines are potentially capable of brusk and long range manual; particles of < 5 μm readily penetrates the airways all the way downwardly to the alveolar space, and particles of < ten μm readily penetrates below the glottis (7) ii) large droplets of diameters > twenty μm refer to those that follow a more ballistic trajectory (i.e. falling more often than not under the influence of gravity), where the droplets are too large to follow inhalation airflow streamlines. For these particle sizes, for example, surgical masks would be effective, as they volition act as a direct physical bulwark to droplets of this size that are also large to exist inhaled into the respiratory tract around the sides of the mask (which are not close-fitting); three) 'intermediate particles' of diameters 10–20 μm, volition share some properties of both small and large droplets, to some extent, but settle more quickly than particles < 10 μm and potentially behave a smaller infectious dose than big (> xx μm) droplets.
'Aerosols' would besides include 'droplet nuclei' which are small particles with an aerodynamic diameter of ten μm or less, typically produced through the process of rapid desiccation of exhaled respiratory droplets [5, six]. Nevertheless, in some situations, such as where there are strong ambient air cantankerous-flows, for example, larger aerosol can bear similar aerosols with the potential to transmit infection via this route (see next section below).
Several properties can exist inferred from this, for instance the penetration of the lower respiratory tract (LRT), every bit at greater than ten μm diameter, penetration beneath the glottis rapidly diminishes, as does any potential for initiating an infection at that site. Similarly, any such potential for depositing and initiating an LRT infection is less probable to a higher place a droplet diameter of 20 μm, as such big particles will probably impact onto respiratory epithelial mucosal surfaces or be trapped by cilia earlier reaching the LRT [vi].
The Infectious Diseases Society of America (IDSA) has proposed a scheme that is essentially equivalent [vii], defining "respirable particles" as having a bore of 10 μm or less; and "inspirable particles" equally having a bore betwixt 10 μm and 100 μm, about all of which are deposited in the upper airways. Some authors have proposed the term "fine aerosols", consisting of particles of 5 μm or less, just this has been in function dictated by constraints from measurement instruments [8]. Several authors lump together manual by either large droplets or aerosol-sized particles every bit "airborne transmission" [9], or use "aerosol transmission" to describe pathogens that tin cause disease via inspirable particles of whatever size [10].
However, we call up that it is of import to maintain a distinction between particles of < 10 μm and larger particles, because of their significant qualitative differences including suspension time, penetration of different regions of the airways and requirements for dissimilar PPE. In this commentary, nosotros use the common convention of "airborne manual" to mean manual by droplets-size particles of < 10 μm.
If the infected patients produce infectious droplets of varying sizes by breathing, coughing or sneezing, manual betwixt individuals by both short-range large droplets and airborne small-scale droplet nuclei are both possible, depending on the distance from the patient source. Effigy i illustrates these potential routes of short and long-range airborne manual, every bit well as the downstream settling of such droplets onto surfaces (fomites). From such fomites, they may be touched and transported by hands to be self-inoculated into mucosal membranes due east.m. in the eyes, nose and mouth) to cause infection, depending on the survival characteristics of individual pathogens on such surfaces, and the susceptibility (related to bachelor, compatible prison cell receptors) of the different exposed tissues to infection by these pathogens.
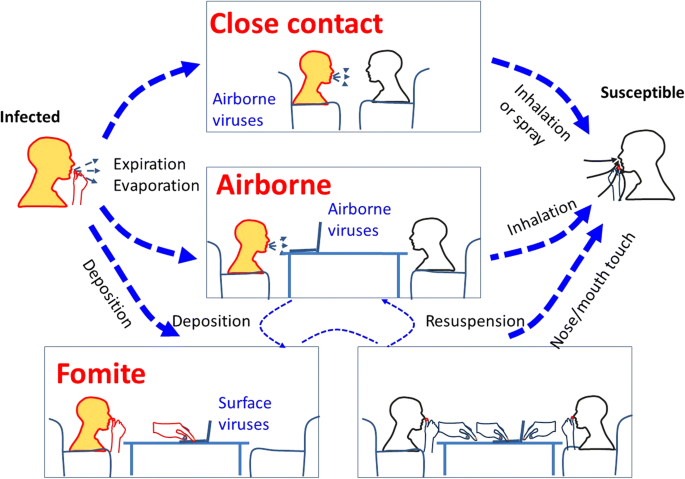
An analogy of diverse possible transmission routes of respiratory infection betwixt an infected and a susceptible individual. Both close range (i.east. conversational) airborne transmission and longer range (over several meters) manual routes are illustrated here. The orangish caput colour represents a source and the white head colour a potential recipient (with the bottom correct panel indicating that both heads are potential recipients via self-inoculation from contaminated surface fomite sources). Hither 'Expiration' also includes normal breathing exhalation, as well every bit coughing and/or sneezing airflows. Airborne aerosol can then settle on surfaces (fomites) from where they can be touched and carried on hands leading to further self-inoculation routes of transmission
For instance, when the infectious dose (the number of infectious agents required to crusade disease) of an organism is depression, and where large numbers of pathogen-laden droplets are produced in crowded conditions with poor ventilation (in infirmary waiting rooms, in lecture theatres, on public transport, etc.), explosive outbreaks can still occur, even with pathogens whose airborne manual capacity is controversial, eastward.one thousand. the spread of influenza in a grounded airplane where multiple secondary cases were observed in the absenteeism of whatsoever ventilation [11].
The more mechanistic approaches (i.e. arguing from the more cardinal physical and dynamic behavior of pocket-sized versus larger particle and droplet sizes in the absence of any biological interactions) to classifying which pathogens are likely to transmit via the airborne road have been published in various means over the years [12,13,14,15,16,17], only may have to exist considered in combination with epidemiological and environmental information to make a convincing argument about the potential for the airborne transmissibility of any particular agent – and the number of possible potential exposure scenarios is virtually unlimited).
The importance of ambient airflows and the of aerosols
One should notation that "droplets" is essentially a relative and not an absolute term. A larger droplet tin remain airborne for longer if ambient airflows tin sustain this suspension for longer, east.g. in some potent cross-menstruum or natural ventilation environments, where ventilation-induced airflows tin can propagate suspended pathogens effectively enough to cause infection at a considerable altitude abroad from the source.
One of the standard rules (Stoke's Law) practical in engineering calculations to estimate the suspension times of droplets falling under gravity with air resistance, was derived assuming several conditions including that the ambience air is still [thirteen,14,15,16,17]. So actual suspension times will be far higher where there are significant cantankerous-flows, which is oft the case in healthcare environments, e.g. with doors opening, bed and equipment move, and people walking back and forth, constantly. Conversely, interruption times, even for smaller droplet nuclei, can exist greatly reduced if they come across a significant downdraft (e.thousand. if they pass under a ceiling supply vent). In addition, the caste of airway penetration, for unlike particle sizes, likewise depends on the menstruum rate.
In the field of dentistry and orthopedics, where high-powered electric tools are used, even bloodborne viruses (such equally human being immunodeficiency virus – HIV, hepatitis B and hepatitis B viruses) tin become airborne when they are contained in high velocity blood splatter generated past these instruments [eighteen, 19]. Yet, whether they tin crusade efficient transmission via this route is more debatable. This illustrates another point, that although some pathogens can be airborne in certain situations, they may not necessarily transmit infection and cause disease via this route.
Outline
Over fourth dimension, for a pathogen with a truly predominant airborne transmission route, eventually sufficient numbers of published studies volition demonstrate its truthful nature [13]. If there are ongoing contradictory findings in multiple studies (equally with influenza virus), it may exist more likely that the various transmission routes (straight/indirect contact, short-range droplet, long-, and fifty-fifty short-range airborne droplet nuclei) may predominate in different settings [xvi, 20], making the airborne route for that particular pathogen more of an opportunistic pathway, rather than the norm [21]. Several examples may make this clearer.
The selected pathogens and supporting literature summarized below are for illustrative purposes only, to demonstrate how specific studies take impacted the way nosotros consider such infectious agents equally potentially airborne and 'aerosol-transmissible'. It is not intended to exist a systematic review, merely rather to prove how our thinking may change with additional studies on each pathogen, and how the acceptance of "aerosol manual" for unlike pathogens did not always followed a consistent approach.
Results and give-and-take
Chickenpox
Chickenpox is a febrile, vesicular rash affliction acquired by varicella zoster virus (VZV), a lipid-enveloped, double-stranded DNA virus, and a member of the Herpesviridae family.
For chickenpox, the bear witness appears to exist mainly epidemiological and clinical, though this has appeared to be sufficient to classify varicella zoster virus (VZV) as an airborne agent. Studies on VZV accept shown that the virus is clearly able to travel long distances (i.east. up to tens of meters abroad from the index case, to spread between isolation rooms and other ward areas continued by corridors, or within a household) to cause secondary infections and/or settle elsewhere in the surroundings [22,23,24]. In add-on, Tang et al. [25] showed that airborne VZV could leak out of isolation rooms transported past induced environmental airflows to infect a susceptible HCW, near probable via the direct inhalation route.
Measles
Measles (also known as rubeola) is a delirious, rash illness caused by the measles virus, a lipid-enveloped, single-stranded, negative-sense RNA virus, and a member of the Paramyxoviridae family.
For measles several studies examined a more mechanistic airflow dynamical explanation (i.e. based upon the central physics and behaviour of airborne particles) for the chief transmission route involved in several measles outbreaks [26], including that of Riley and colleagues who used the concept of 'quanta' of infection [27]. Later, two other outbreaks in outpatient clinics included retrospective airflow dynamics assay, providing more evidence for the transmissibility of measles via the airborne route [28, 29].
Tuberculosis
Tuberculosis is a localized or systemic, but most ofttimes respiratory bacterial illness acquired by mycobacteria belonging to the Mycobacterium tuberculosis complex.
For tuberculosis (TB), definitive experimental evidence of airborne transmission being necessary and sufficient to cause affliction was provided in a series of guinea-hog experiments [30, 31], which has been repeated more than recently in a slightly different clinical context [32]. Numerous other outbreak reports have confirmed the transmissibility of TB via the airborne route [33,34,35], and interventions specifically targeting the airborne transmission route have proven effective in reducing TB transmission [36].
Smallpox
Smallpox is a now eradicated, febrile, vesicular rash and disseminated illness, caused by a complex, double-stranded Deoxyribonucleic acid orthopoxvirus (Poxviridae family unit), which can nowadays clinically in two forms, as variola major or variola minor.
For smallpox, a contempo comprehensive, retrospective analysis of the literature by Milton has suggested an of import contribution of the airborne transmission road for this infection [37]. Although various air-sampling and creature manual studies were also reviewed, Milton likewise emphasized clinical epidemiological studies where not-airborne transmission routes alone could not account for all the observed smallpox cases.
At least ane well-documented infirmary outbreak, involving 17 cases of smallpox, could merely exist explained by bold the aerosol spread of the virus from the alphabetize case, over several floors. Retrospective smoke tracer experiments further demonstrated that airborne virus could easily spread to patients on different floors via open windows and connecting corridors and stairwells in a pattern roughly replicating the location of cases [38].
Emerging coronaviruses: Severe acute respiratory syndrome (SARS), center-e respiratory syndrome (MERS)
Coronaviruses are lipid-enveloped, unmarried-stranded positive sense RNA viruses, belong to the genus Coronavirus and include several relatively benign, seasonal, common cold viruses (229E, OC43, NL63, HKU-1). They also include two new more virulent coronaviruses: astringent acute respiratory syndrome coronavirus (SARS-CoV), which emerged in the human being population in 2003; and Heart-East Respiratory Syndrome coronavirus (MERS-CoV), which emerged in humans during 2012.
For SARS-CoV, several thorough epidemiological studies that include retrospective airflow tracer investigations are consistent with the hypothesis of an airborne manual route [39,xl,41]. Air-sampling studies accept also demonstrated the presence of SARS-CoV nucleic acid (RNA) in air, though they did non examination viability using viral culture [42].
Although several studies compared and contrasted SARS and MERS from clinical and epidemiological angles [43,44,45], the predominant transmission mode was not discussed in detail, if at all. Several other studies practise mention the potential for airborne transmission, when comparing potential routes of infection, merely mainly in relation to super-spreading events or "aerosolizing procedures"such as broncho-alveolar lavage, and/or a potential route to take into consideration for precautionary infection control measures [46,47,48]. Yet, from the various published studies, for both MERS and SARS, information technology is arguable that a proportion of transmission occurs through the airborne route, although this may vary in different situations (e.g. depending on host, and environmental factors). The contribution from asymptomatic cases is also uncertain [49].
For both SARS and MERS, LRT samples offer the best diagnostic yield, often in the absence of any detectable virus in upper respiratory tract (URT) samples [fifty,51,52]. Furthermore, infected, symptomatic patients tend to develop severe LRT infections rather than URT disease. Both of these aspects indicate that this is an airborne amanuensis that has to penetrate directly into the LRT to preferentially replicate there before causing disease.
For MERS-CoV specifically, a contempo study demonstrated the absence of expression of dipeptidyl peptidase 4 (DPP4), the identified receptor used by the virus, in the cells of the homo URT. The search for an alternating receptor was negative [53]. Thus, the human URT would seem little or not-permissive for MERS-CoV replication, indicating that successful infection can but upshot from the penetration into the LRT via direct inhalation of accordingly sized 'droplet nuclei'-similar' particles. This makes any MERS-CoV transmission leading to MERS disease provisional on the presence of virus-containing droplets small plenty to be inhaled into the LRT where the virus can replicate.
Influenza
Influenza is a seasonal, oft febrile respiratory illness, caused by several species of influenza viruses. These are lipid-enveloped, single-stranded, negative-sense, segmented RNA viruses belonging to the Orthomyxoviridae family. Currently, influenza is the only common seasonal respiratory virus for which licensed antiviral drugs and vaccines are bachelor.
For human influenza viruses, the question of airborne versus large droplet transmission is maybe most controversial [54,55,56,57]. In experimental inoculation experiments on homo volunteers, aerosolized flu viruses are infectious at a dose much lower than by nasal instillation [58]. The likely answer is that both routes are possible and that the importance and significance of each road will vary in dissimilar situations [16, 20, 21].
For example, tighter control of the environment may reduce or foreclose airborne transmission past: 1) isolating infectious patients in a single-bed, negative force per unit area isolation room [25]; 2) decision-making ecology relative humidity to reduce airborne influenza survival [59]; iii) reducing exposure from aerosols produced by patients through coughing, sneezing or breathing with the use of personal protective equipment (wearing a mask) on the patient (to reduce source emission) and/or the healthcare worker (to reduce recipient exposure) [threescore]; 4) carefully controlling the utilize and exposure to whatsoever respiratory assistance devices (high-catamenia oxygen masks, nebulizers) by only allowing their use in designated, containment areas or rooms [61]. The airflows being expelled from the side vents of oxygen masks and nebulisers will comprise a mixture of patient exhaled air (which could be conveying airborne pathogens) and incoming high period oxygen or air carrying nebulized drugs. These vented airflows could so human action equally potential sources of airborne pathogens.
Numerous studies have shown the emission of influenza RNA from the exhaled jiff of naturally influenza-infected human subjects [62,63,64,65,66] and accept detected influenza RNA in environmental air [67,68,69]. More recently, some of these studies have shown the absence of [70], or significantly reduced numbers of feasible viruses in air-samples with loftier flu RNA levels (equally tested by PCR) [66, 71, 72]. The low number of infectious particles detected is currently hard to interpret as culture methods are inherently less sensitive than molecular methods such as PCR, and the actual operation of air-sampling itself, through shear-stress related impairment to the virions, also causes a drop in infectivity in the collected samples. This may pb to underestimates of the amount of live virus in these ecology aerosols.
An additional variable to consider is that some animal studies have reported that different strains of influenza virus may vary widely in their capacity for aerosol transmission [73].
In some earlier articles that discuss the predominant mode of influenza virus transmission [74,75,76,77,78], these same questions are addressed with mixed conclusions. Nearly of the evidence described to support their views was more clinical and epidemiological, and included some animal and human being volunteer studies, rather than physical and mechanistic. Yet, this mixed picture of transmission in dissimilar circumstances is probably the almost realistic.
It is noteworthy that several infections currently accepted every bit airborne-transmitted, such as measles, chickenpox or TB present, in their classical form, an unmistakable and pathognomonic clinical motion-picture show. In contrast the clinical pic of flu virus infection has a large overlap with that of other respiratory viruses, and mixed outbreaks have been documented [79]. Thus, a prevalent misconception in the field has been to report 'respiratory viruses' equally a group. Yet, given that these viruses belong to unlike genera and families, accept different chemic and physical properties and differing viral characteristics, it is unwise and inaccurate to assume that any conclusions almost one virus can be practical to another, eastward.k. in a Cochrane review of 59 published studies on interventions to reduce the spread of respiratory viruses, there were actually merely two studies specifically about flu viruses [fourscore]. Every bit the authors themselves pointed out, no conclusion specific to influenza viruses was possible.
While many airborne infections are highly contagious, this is non, strictly speaking, part of the definition. Even then, the lower contagiousness of influenza compared to, say, measles has been invoked equally an argument against a significant contribution of airborne transmission. However, it should be noted that a feature of influenza virus infections is that the incubation time (typically 1–2 days) is much shorter than its duration of shedding. This allows for the possibility that a susceptible person will be exposed during an outbreak to several different infectious cases belonging to more than one generation in the outbreak. This multiple exposure and telescoping of generations may event in an underestimate of influenza virus transmissibility, as fewer secondary cases volition be assigned to a known index case, when in fact the number of secondary cases per index could exist much higher. For example, it is known that in some settings a single alphabetize case can infect a large number of people, eastward.g. 38 in an outbreak on an Alaska Airlines flying [xi].
Ebola
Ebola is a viral hemorrhagic fever associated with a very high mortality, caused past the Ebola viruses; these are enveloped single-strand, negative-sense RNA viruses comprising five species within the family unit Filoviridae. Four Ebola species have been implicated in human diseases; the nearly widespread outbreak, besides the near contempo, was caused by Ebola Zaire in Due west Africa in 2013–2016. The transmission of Ebola viruses has been reviewed in depth by Osterholm et al. (four). These authors noted the broad tissue tropism, equally well as the high viral load reached during illness and the low infectious dose, from which it appears inescapable that more than one mode of transmission is possible.
Regarding aerosol manual, concerns are raised by several documented instances of manual of Ebola Zaire in laboratory settings between animals without directly contact [81, 82] (as well reviewed in [4]). Experimental infections of Rhesus monkeys by Ebola Zaire using aerosol infection has been shown to be highly effective [83, 84] and this experimental procedure has in fact been used as infectious challenge in Ebola vaccine studies [85, 86]. Rhesus monkeys infected by aerosol exposure reliably adult disseminated, fatal infection substantially similar to that caused by parenteral infection with the improver of involvement of the respiratory tract. Autopsies showed pathological findings in the respiratory tract and respiratory lymphoid system in animals infected by the droplets route that are not found in animals infected parenterally [83, 84].
Such respiratory pathological lesions accept not been reported in human autopsies of Ebola cases, but equally noted by Osterholm et al. [4], there have been few human autopsies of Ebola cases, arguably too few to confidently dominion out any possibility of disease caused by the aerosol route. The precautionary principle would therefore dictate that aerosol precautions be used for the intendance of infected patients, and especially considering that infection of the respiratory tract in such patients is not necessary to create an aerosol chance: Ebola viruses reach a very high titer in blood or other actual fluids during the affliction [87, 88] and aerosolization of claret or other fluids would create a significant airborne transmission run a risk.
Conclusions
In summary, despite the various mechanistic arguments about which organisms can exist potentially airborne and therefore aerosol-transmissible, ultimately, the primary deciding factor appears to be how many studies using various differing approaches: empirical (clinical, epidemiological), and/or experimental (e.g. using animal models), and/or mechanistic (using airflow tracers and air-sampling) methods, reach the same consensus opinion. Over time, the scientific community will eventually form an impression of the predominant transmission route for that specific agent, even if the conclusion is one of mixed transmission routes, with different routes predominating depending on the specific situations. This is the example for influenza viruses, and is likely the near realistic.
Some bacterial and viral infections that have more than ane style of transmission are also anisotropic, like anthrax, plague, tularemia and smallpox: the severity of the disease varies depending on the mode of transmission [37, 89]. Older experimental infection experiments on volunteers suggest that this is the case for flu, with transmission past aerosols existence associated with a more severe affliction [xiv, 90], and some more than contempo field observations are consistent with this concept [57]. For anisotropic agents, fifty-fifty if a mode of manual (e.thou. aerosols) accounts for only a minority of cases, interruption of that route of manual may be required if information technology accounts for the near astringent cases.
Abbreviations
- LRT:
-
lower respiratory tract
- MERS-CoV:
-
Centre Eastward Respiratory Syndrome-associated coronavirus
- PCR:
-
polymerase chain reaction
- RNA:
-
ribonucleic acid
- SARS-CoV:
-
severe acute respiratory syndrome-associated coronavirus
- TB:
-
tuberculosis
- URT:
-
upper respiratory tract
- VZV:
-
varicella zoster virus
References
-
CIDRAP (Center for Communicable diseases Research and Policy). Commentary: Protecting health workers from airborne MERS-CoV—learning from SARS https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html. Accessed 9 August 2017.
-
Kim SH, Chang SY, Sung M, et al. Extensive viable Centre E respiratory syndrome (MERS) coronavirus contagion in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–9.
-
CIDRAP (Center for Infectious disease Inquiry and Policy). Commentary: Health workers need optimal respiratory protection for Ebola https://world wide web.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html. Accessed 9 Baronial 2017.
-
Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong 1000, Spud FA, et al. Transmission of Ebola viruses: what we know and what nosotros do not know. MBio. 2015;6:e00137.
-
Cole EC, Cook CE. Label of infectious aerosols in health care facilities: an aid to effective technology controls and preventive strategies. Am J Infect Control. 1998;26:453–64.
-
Hinds WC. Droplets technology. 2d ed. New York: John Wiley & Sons; 1999.
-
Infectious Diseases Society of America (ISDA). Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel: Update 2010. Chapter: 2 Agreement the Risk to Healthcare Personnel. 2010. https://www.nap.edu/read/13027/chapter/four#thirty
-
Yan J, Grantham M, Pantelic J, Bueno de Mesqita PJ, Albert B, Liu F, et al. Infectious virus in exhaled breath of symptomatic seasonal flu cases from a college community. Proc Natl Acad Sci U S A. 2018;115:1081–86.
-
Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne manual of influenza a/H5N1 virus between ferrets. Science. 2012;336:1534–41.
-
Centers for Disease Control and prevention (CDC). Approaches to Better Understand Human Influenza Transmission. 2010. https://www.cdc.gov/influenzatransmissionworkshop2010/
-
Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–vi.
-
Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare bounds. J Hosp Infect. 2006;64:100–xiv.
-
Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets tin move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–25.
-
Li Y, Leung GM, Tang JW, Yang X, Chao CY, Lin JZ, et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air. 2007;17:2–xviii.
-
Jones RM, Brosseau LM. Aerosol transmission of communicable diseases. J Occup Environ Med. 2015;57:501–8.
-
Liu L, Li Y, Nielsen PV, Wei J, Jensen RL. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27:452–62.
-
Aliabadi AA, Rogak SN, Bartlett KH, Green SI. Preventing airborne affliction transmission: review of methods for ventilation Design in Health Care Facilities. Adv Prev Med. 2011;2011:124064.
-
Jewett DL, Heinsohn P, Bennett C, Rosen A, Neuilly C. Blood-containing aerosols generated by surgical techniques: a possible infectious gamble. Am Ind Hyg Assoc J. 1992;53:228–31.
-
Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–37.
-
Wei J, Li Y. Airborne spread of infectious agents in the indoor surroundings. Am J Infect Control. 2016;44(9 Suppl):S102–viii.
-
Roy CJ, Milton DK. Airborne transmission of catching infection--the elusive pathway. Due north Engl J Med. 2004;350:1710–2.
-
Asano Y, Iwayama S, Miyata T, Yazaki T, Ozaki T, Tsuzuki K, et al. Spread of varicella in hospitalized children having no direct contact with an indicator zoster instance and its prevention by a live vaccine. Biken J. 1980;23:157–61.
-
Gustafson TL, Lavely GB, Brawner ER Jr, Hutcheson RH Jr, Wright PF, Schaffner W. An outbreak of airborne nosocomial varicella. Pediatrics. 1982;70:550–6.
-
Suzuki Thousand, Yoshikawa T, Ihira G, Ohashi Grand, Suga S, Asano Y. Spread of varicella-zoster virus DNA to the surroundings from varicella patients who were treated with oral acyclovir. Pediatr Int. 2003;45:458–lx.
-
Tang JW, Eames I, Li Y, Taha YA, Wilson P, Bellingan Yard, et al. Door-opening motion tin can potentially lead to a transient breakup in negative-pressure isolation weather: the importance of vorticity and buoyancy airflows. J Hosp Infect. 2005;61:283–six.
-
Wells WF, Wells WM, Wilder TS. The environmental command of epidemic contagion. I. An epidemiologic study of radiant disinfection of air in day schools Am J Hyg. 1942;35:97–121.
-
Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107:421–32.
-
Bloch AB, Orenstein WA, Ewing WM, Spain WH, Mallison GF, Herrmann KL, et al. Measles outbreak in a pediatric practice: airborne manual in an role setting. Pediatrics. 1985;75:676–83.
-
Remington PL, Hall WN, Davis IH, Herald A, Gunn RA. Airborne manual of measles in a md's office. JAMA. 1985;253:1574–seven.
-
Riley RL, Mills CC, Nyka W, Weinstock North, Story PB, Sultan LU, Riley MC, Wells WF. Aerial Dissemination of pulmonary tuberculosis a two year study of contagion in a tuberculosis ward. Am J Hyg. 1959;lxx:185–96.
-
Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–25.
-
Escombe AR, Moore DA, Gilman RH, Pan Due west, Navincopa M, Ticona Due east, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5:e188.
-
Houk VN. Spread of tuberculosis via recirculated air in a naval vessel: the Byrd written report. Ann N Y Acad Sci. 1980;353:10–24.
-
Hutton Dr., Stead WW, Cauthen GM, Bloch AB, Ewing WM. Nosocomial manual of tuberculosis associated with a draining abscess. J Infect Dis. 1990;161:286–95.
-
Kenyon TA, Valway SE, Ihle WW, Onorato IM, Castro KG. Manual of multidrug-resistant Mycobacterium tuberculosis during a long airplane flying. North Engl J Med. 1996;334:933–eight.
-
Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, et al. Upper-room ultraviolet lite and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43.
-
Milton DK. What was the primary way of smallpox transmission? Implications for biodefense Front Jail cell Infect Microbiol. 2012;2:150.
-
Wehrle PF, Posch J, Richter KH, Henderson DA. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Balderdash World Health Organ. 1970;43:669–79.
-
Wong TW1, Lee CK, Tam West, Lau JT, Yu TS, Lui SF, et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276.
-
Olsen SJ, Chang HL, Cheung TY, Tang AF, Fisk TL, Ooi SP, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–22.
-
Yu IT, Li Y, Wong TW, Tam Due west, Chan AT, Lee JH, et al. Evidence of airborne manual of the astringent astute respiratory syndrome virus. Due north Engl J Med. 2004;350:1731–9.
-
Booth TF1, Kournikakis B, Bastien North, Ho J, Kobasa D, Stadnyk L, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and ecology contagion in SARS outbreak units. J Infect Dis. 2005;191:1472–1477.
-
Assiri A1, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Center Eastward respiratory syndrome coronavirus affliction from Saudi Arabia: a descriptive report. Lancet Infect Dis. 2013;13:752–761.
-
Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–41.
-
Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: astringent astute respiratory syndrome coronavirus and Heart East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014;27:411–7.
-
Guery B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a written report of nosocomial manual. Lancet. 2013;381:2265–72.
-
Mailles A, Blanckaert K, Chaud P, van der Werf S, Lina B, Caro V, et al. Get-go cases of Middle Eastward Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-man transmission, France, May 2013. Euro Surveill. xiii;18(24).
-
Chowell K, Abdirizak F, Lee Southward, Lee J, Jung East, Nishiura H, et al. Manual characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210.
-
Omrani As, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA, Albarrak AM. A family cluster of Center East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–72.
-
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72.
-
Poissy J, Goffard A, Parmentier-Decrucq E, Favory R, Kauv M, Kipnis E, et al. Kinetics and pattern of viral excretion in biological specimens of ii MERS-CoV cases. J Clin Virol. 2014;61:275–viii.
-
Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Assiri A, Alhakeem RF, Albarrak A, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Eye East respiratory syndrome. J Infect Dis. 2014;210:1590–4.
-
Widagdo W, Raj VS, Schipper D, Kolijn One thousand, van Leenders GJ, Bosch BJ, et al. Differential expression of the MERS-coronavirus receptor in the upper respiratory tract of humans and dromedary camels. J Virol. 2016;90:4838–42.
-
Tellier R. Review of aerosol transmission of influenza a virus. Emerg Infect Dis. 2006;12:1657–62.
-
Tellier R. Droplets transmission of influenza a virus: a review of new studies. J R Soc Interface. 2009;vi(Suppl 6):S783–90.
-
Cowling BJ. Airborne manual of flu: implications for control in healthcare and customs settings. Clin Infect Dis. 2012;54:1578–80.
-
Cowling BJ, Ip DK, Fang VJ, Suntarattiwong P, Olsen SJ, Levy J, et al. Aerosol transmission is an important mode of influenza a virus spread. Nat Commun. 2013;four:1935.
-
Alford RH, Kasel JA, Gerone PJ, Knight V. Homo flu resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800–4.
-
Tang JW. The outcome of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;6(Suppl 6):S737–46.
-
United states Centers for Disease Control and Prevention (CDC). Acting Guidance for the Use of Masks to Control Influenza Transmission. https://world wide web.cdc.gov/flu/professionals/infectioncontrol/maskguidance.htm. Accessed 9 August 2017.
-
O'Neil CA,Li J,Leavey A,Wang Y,Hink M, Wallace One thousand, et al. Characterization of Aerosols Generated During Patient Care Activities. Clin Infect Dis. 2017; doi.org/10.1093/cid/cix535
-
Fabian P, McDevitt JJ, DeHaan WH, Fung RO, Cowling BJ, Chan KH, et al. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3:e2691.
-
Stelzer-Braid Due south, Oliver BG, Blazey AJ, Argent E, Newsome TP, Rawlinson WD, et al. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81:1674–9.
-
Lindsley WG, Noti JD, Blachere FM, Thewlis RE, Martin SB, Othumpangat Due south, et al. Viable influenza a virus in airborne particles from human coughs. J Occup Environ Hyg. 2015;12:107–13.
-
Lindsley WG, Blachere FM, Beezhold DH, Thewlis RE, Noorbakhsh B, Othumpangat S, et al. Feasible influenza a virus in airborne particles expelled during coughs vs. Exhalations Influenza Other Respir Viruses. 2016;10:404–xiii.
-
Yan J, Grantham One thousand, Pantelic J, Bueno de Mesquita PJ, Albert B, Liu F, Ehrman S, Milton DK. EMIT Consortium Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a higher community. Proc Natl Acad Sci U South A, 2018;115:1081–6.
-
Yang W, Elankumaran Southward, Marr LC. Concentrations and size distributions of airborne influenza a viruses measured indoors at a wellness Heart, a 24-hour interval-care Center and on aeroplanes. J R Soc Interface. 2011;8:1176–84.
-
Bischoff WE, Swett K, Leng I, Peters TR. Exposure to flu virus aerosols during routine patient intendance. J Infect Dis. 2013;207:1037–46.
-
Leung NH, Zhou J2, Chu DK, Yu H, Lindsley WG, Beezhold DH, et al. Quantification of Influenza Virus RNA in Aerosols in Patient Rooms PLoS One 2016;eleven:e0148669.
-
Tang JW, Gao CX, Cowling BJ, Koh GC, Chu D, Heilbronn C, et al. Absenteeism of detectable flu RNA transmitted via aerosol during various man respiratory activities--experiments from Singapore and Hong Kong. PLoS One. 2014;9:e107338.
-
Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human being exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;nine:e1003205.
-
Hatagishi E, Okamoto G, Ohmiya South, Yano H, Hori T, Saito W, et al. Institution and clinical applications of a portable system for capturing influenza viruses released through coughing. PLoS One. 2014;9:e103560.
-
Koster F, Gouveia Thou, Zhou Y, Lowery K, Russell R, MacInnes H, et al. Exhaled droplets transmission of pandemic and seasonal H1N1 flu viruses in the ferret. PLoS I. 2012;seven:e33118.
-
Goldmann DA. Transmission of viral respiratory infections in the dwelling house. Pediatr Infect Dis J. 2000;19(x Suppl):S97–102.
-
Goldmann DA. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg Infect Dis. 2001;7:249–53.
-
Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute infirmary setting. Lancet Infect Dis. 2002;2:145–55.
-
Bridges CB, Kuehnert MJ, Hall CB. Manual of flu: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–101.
-
Hall CB. The spread of influenza and other respiratory viruses: complexities and conjectures. Clin Infect Dis. 2007;45:353–ix.
-
Mathur U, Bentley DW, Hall CB. Concurrent respiratory syncytial virus and influenza a infections in the institutionalized elderly and chronically sick. Ann Intern Med. 1980;93:49–52.
-
Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical intervention to interrupt and reduce the spread of respiratory viruses: a Cochrane review. Wellness Technol Assess. 2010;14:347–476.
-
Jaax N, Jarhlign P, Gesibert T, Geisbert Southward, Steele 1000, McKee 1000, et al. Manual of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet. 1995;346:1669–71.
-
Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith Thousand, Kobinger K. Manual of Ebola virus from pigs to non-human primates. Sci Rep. 2012. https://doi.org/10.1038/srep00811.
-
Twenhafel NA, Mattix ME, Johnson JC, Robinson CG, Pratt WD, Cashman KA, et al. Pathology of experimental droplets Zaire ebolavirus infection in rhesus macaques. Vet Pathol. 2012;50:514–29.
-
Johnson E, Jaax N, White J, Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Path. 1995;76:227–36.
-
Herbert As, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, Zak SE, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and droplets challenge with ebolavirus. J Virol. 2013;87:4952–64.
-
Pratt WD, Wang D, Nichols DK, Luo One thousand, Woraratanadharm J, Dye JM, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single circuitous adenovirus vector. Clin Vaccine Immunol. 2010;17:572–81.
-
Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent 1000, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting an assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–41.
-
Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, et al. A instance of severe Ebola virus infection complicated past gram-negative septicemia. N Engl J Med. 2014;371:2394–401.
-
Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278:399–411.
-
Little JW, Douglas RG Jr, Hall WJ, Roth FK. Attenuated influenza produced past experimental intranasal inoculation. J Med Virol. 1979;3:177–88.
Acknowledgements
None.
Funding
None required.
Availability of data and materials
All studies cited/discussed are already published and in the public domain – some require the relevant journal subscriptions for access.
Disclaimer
Delight note that the views expressed here are solely those of the authors and are not representative of the institutions to which they are affiliated.
Author information
Affiliations
Contributions
JWT, RT, BJC developed the original concept and outline of the commodity; YL contributed the figures and some additional related text; all authors critically reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Respective author
Ideals declarations
Ethics approval and consent to participate
Non required. No individual patient information is included. Only previously published papers are discussed.
Consent for publication
Not applicable.
Competing interests
None of the authors have any competing interests to declare.
Publisher's Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Admission This article is distributed under the terms of the Artistic Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted apply, distribution, and reproduction in any medium, provided you give advisable credit to the original author(s) and the source, provide a link to the Artistic Commons license, and signal if changes were made. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/goose egg/1.0/) applies to the data made bachelor in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Tellier, R., Li, Y., Cowling, B.J. et al. Recognition of droplets transmission of infectious agents: a commentary. BMC Infect Dis 19, 101 (2019). https://doi.org/10.1186/s12879-019-3707-y
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12879-019-3707-y
Keywords
- Aerosol
- Airborne
- Droplet
- Transmission
- Infection
Source: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3707-y
Posted by: westwelition.blogspot.com


0 Response to "Which Of The Following Statements Is True About Modes Of Transmission For Infectious Agents?"
Post a Comment